Kupffer Cells In Liver: Definition, Development, Anatomy, and Functions
Kupffer Cells are specialized macrophages in the liver. Let’s explore the definition, anatomy, and functions of Kupffer cells. Kupffer cells are mammalian bodies’ most abundant tissue macrophages, constituting 80-90%. Also known as Kupffer-Browicz cells or stellate macrophages.
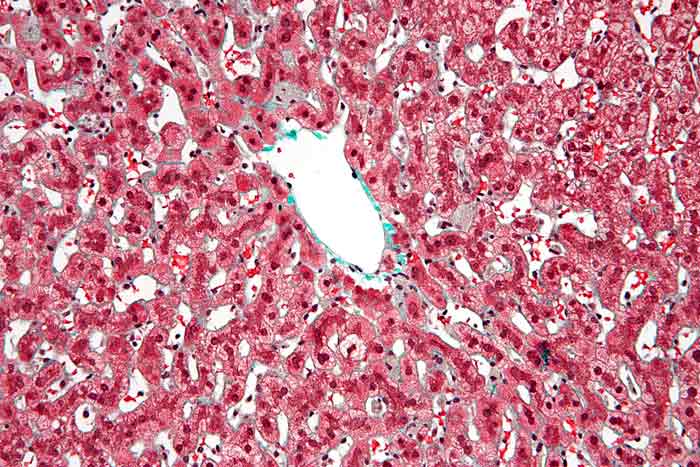
Kupffer Cells: Every day, living organisms like humans are subject to the attack of disease-causing agents called pathogens. The only good thing is that they have a special kind of innate protection referred to as “immunity“.
Jump to:
As the organisms’ complexity level increases, their immunity also becomes more extensive. Humans, for example, have various cells in the body which act to help deter the presence of pathogens.
Coming from the two Greek words “makro” and “phagein” which mean “big” and “to eat” respectively, macrophages[1] are large and specialized cells for destroying and engulfing foreign bodies. Check out the complete history of immunology and its timeline here.
They are usually formed as a response to an infection or an accumulation of dead and damaged cells. Interestingly, macrophages in the body are modified to different structures and forms to adapt to various microorganisms and invaders.
For instance, macrophages in the liver sinusoids are called Kupffer cells.
How to pronounce Kupffer Cells?
What are Kupffer Cells?
In mammalian bodies, Kupffer cells are the most abundant tissue macrophages as they constitute 80-90%[2] of them. Also known as Kupffer-Browicz cells or stellate macrophages, these specialized macrophages are specifically found in the liver.
As identified in the early 1970s, the specialized function of Kupffer cells is mainly due to their peroxidase activity. In biology, this is termed the “tolerogenic” phentotype[3] necessary to prevent undesired immune response to any stimuli.
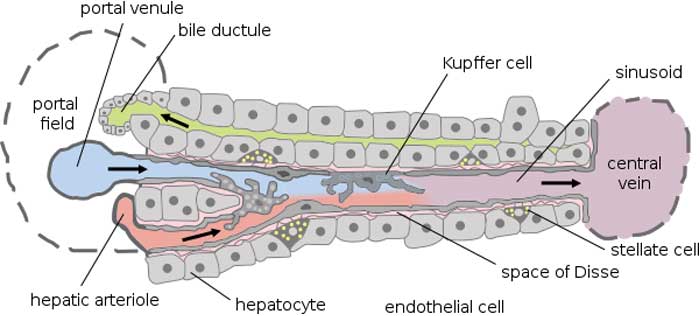
They are identified to contain vacuoles and granules and tubular and vermiform (worm-like) invaginations.
Discovery of Kupffer Cells
-
1876:
 First described in 1876, they were named after the German scientist Karl Wilhelm von Kupffer. This discovery[4] started when von Kupffer observed this so-called “Sternzellen” (the German word for “star cells“) in the liver. By using the gold chloride staining technique, he thought that these cells have functions in the tissue called space of Disse (perisinusoidal connective tissue in liver blood vessels).
First described in 1876, they were named after the German scientist Karl Wilhelm von Kupffer. This discovery[4] started when von Kupffer observed this so-called “Sternzellen” (the German word for “star cells“) in the liver. By using the gold chloride staining technique, he thought that these cells have functions in the tissue called space of Disse (perisinusoidal connective tissue in liver blood vessels).
Twenty-two years later, such an idea was disproved by scientist Tadeusz Browicsz as he correctly identified them as macrophages.
Development of Kupffer Cells
In humans and other mammals, the development[5] of Kupffer cells starts in the yolk sac. Primitive macrophages differentiate into fetal macrophages and then enter the bloodstream. After that, they go to the fetal liver to become mature cells.
Here is an overview of Phagocytosis.
Anatomy of Kupffer Cell
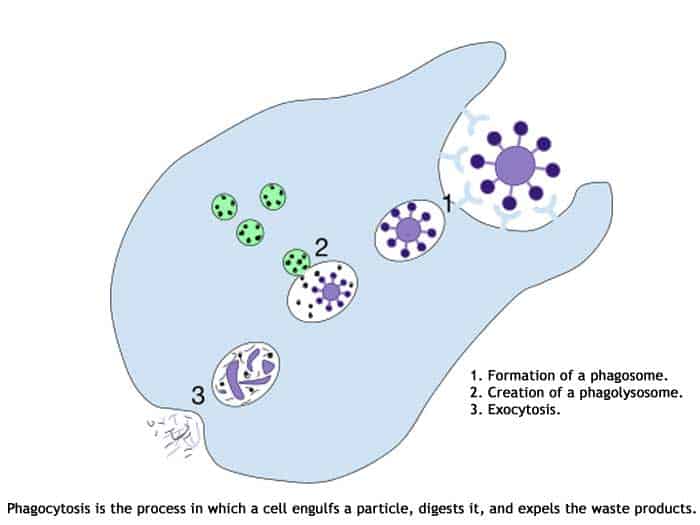
These cells are too minute to be observed individually using a compound microscope. Often, electron microscopy is to observe the Kupffer cells elongated to worm-like to the star-shaped body.
Like any other cells, they have various distributions and arrangements of organelles. Described below are some of them.
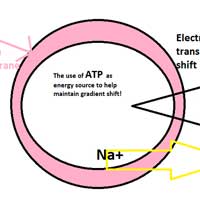
Plasma Membrane
Cytoplasm
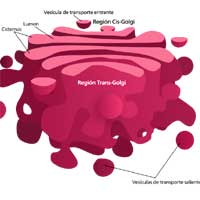
Golgi Bodies

Rough Endoplasmic Reticulum (RER)
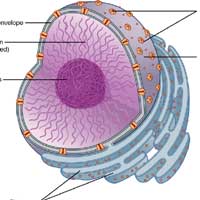
Nucleus
Other Organelles
Functions of Kupffer Cells
Aside from digestion, detoxification, and storage, the liver can function for immunity through the Kupffer cells. The following are ways in which such cells contribute to immune function.
To remove protein complexes and small particles from blood
Together with other cells in the sinusoid endothelium, they are the first line of defense against pathogens[6] entering the liver through the venous portal vein.
- This function is important because the blood that passes through that vein is rich with pathogen-derived products like lipopolysaccharides and proteins.
- Such products must be removed from circulation to prevent systemic immune activation.
To capture and digest microorganisms and worn-out cells
As macrophages, Kupffer cells can engulf and break down[7] old red blood cells that pass through the liver sinusoids.
- In addition, because of their peroxidase activity in the cytoplasm, they can degrade bacterial and other microorganism walls.
To modulate iron homeostasis in the liver
A study published in the Journal of Molecular Medicine (2008)[8] showed that Kupffer cells could control ion homeostasis through the exertion of regulatory signals for the expression of hepcidin.
- Hepcidin is a peptide hormone that primarily controls the entry of iron in the circulatory system of mammals. Interestingly, when hepcidin levels increase (especially during inflammation or immune response), serum iron and the absorption of iron in the gut decreases.
- However, at present, that suppressing molecule produced by Kupffer cells is not yet identified.
To regulate anti-viral immunity during Hepatitis B and C infections
A study published in the Journal of Hepatology revealed that aside from their “barrier” and “janitor” function, they can also be used to inhibit the growth of viral infections in the liver, particularly Hepatitis B and C infections.
- Together with other macrophages, they contribute to the tissue damage of the infected body part. Aside from that, they also regulate fibrosis (thickening of connective tissue), cirrhosis (scarring of the liver), and hepatocellular carcinoma (abnormal growth of liver cells), which all happen during hepatitis.
- However, the exact mechanisms of how Kupffer cells mediate these infections remain unclear and are still up for further studies.
As mentioned above, Kupffer cells exhibit great adaptability depending primarily on their immune environment. But despite medical practices and technology advancements, understanding the exact mechanisms of their functions is still very rudimentary.
Hence, future studies should promote novel insights into the potential of Kupffer cells for therapeutic manipulation. Wouldn’t that be great?
Cite this page
Bio Explorer. (2026, January 4). Kupffer Cells In Liver: Definition, Development, Anatomy, and Functions. https://www.bioexplorer.net/kupffer-cells.html/