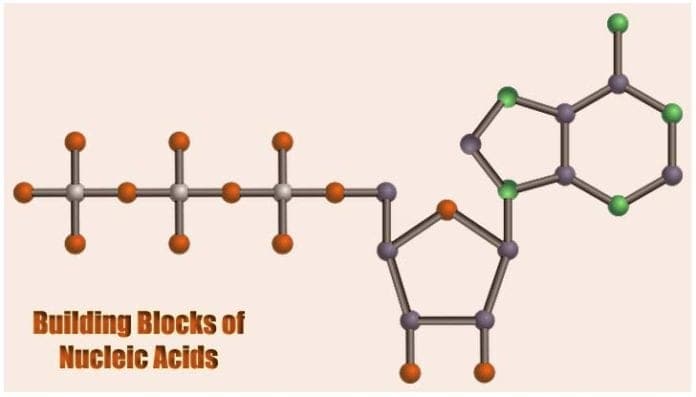
Building Blocks of Nucleic Acids: The study of heredity (the passing of traits from parents to succeeding generations) primarily depends on the understanding of the biochemical properties of the genetic material namely DNA in eukaryotes and bacteria, and RNA in viruses.
It is important to note that some of the essential characteristics of the genetic material in the form of nucleic acids arise from its building blocks: the nucleotides.
Therefore, the knowledge about these small units can serve as critical points to the greater understanding of their role in biological systems.
Below, learn more about the building blocks of nucleic acids, their structures, functions, as well as their importance.
Table of Contents
What Is A Nucleotide?

- Series of these covalent linkages among nucleotide units form the polymer nucleic acids.
- DNA molecules[2] are composed of two strands of these polymers and are coiled in a so-called “double helix“[2]. RNA molecules on the other hand are single stranded and just have some regions where the strand twists.
- American biochemist, Phoebus Levene[3] first coined the term “nucleotide” in the 1900s, long before James Watson and Francis Crick discovered the structure of the DNA.
![]()
Structure of The Nucleotide
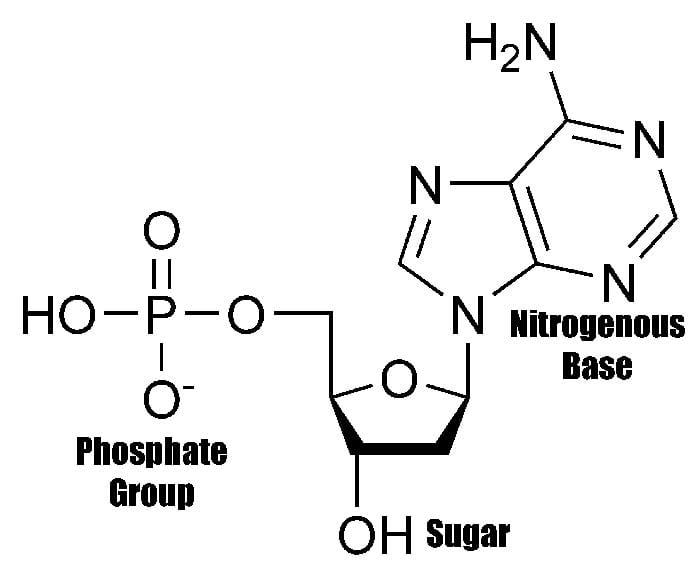
The structure of the nucleotide[4] maximizes bonding potential as it allows same building blocks to be connected to one another through the formation of hydrogen bond between each nucleotide’s nitrogenous base.
Such structure is also efficient in allowing the creation of phosphodiester bonds between the pentose sugar of one nucleotide to the phosphate group of another. These connections, therefore, contribute to giving the DNA’s “double-stranded” ladder-like figure.
- While they seem to be almost indistinguishable, nucleotides and nucleosides can be differentiated through their presence of phosphate groups in their structure; Nucleosides can be phosphorylated to produce nucleotides.
![]()
Building Blocks of Nucleic Acids
The structure of nucleic acids (i.e., DNA) can be likened to a ladder that is made up of alternating steps that are symbolizing its three significant parts: pentose sugar, the phosphate group, and the nitrogenous base.
1. Pentose Sugar
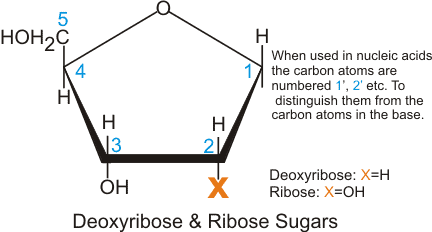
A pentose sugar is a five-carbon sugar that serves as the polymer backbone of DNA and RNA. RNA has the ribose sugar while DNA has the deoxyribose. The two differ regarding the functional group attached to the second carbon position. An -OH group can be found in ribose while deoxyribose has hydrogen instead.
- The difference in the number of oxygen atoms[5] in their structures serves as markers for enzymes to easily distinguish them from each other.
- Also, this functional group difference makes the RNA relatively less stable than DNA. Because of the -OH group, RNA is readily hydrolyzed at basic pH.
![]()
2. Phosphate Group
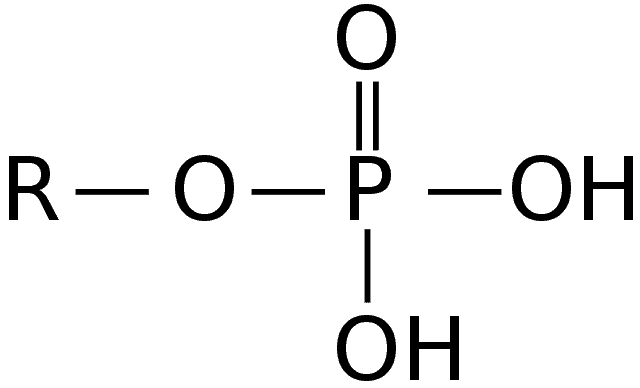
Along with the pentose sugar, the phosphate group makes up the polymer backbone of DNA and RNA. The group is attached to the fifth carbon in place of the hydroxyl group.
- The phosphate group[6] determines the direction of the nucleic acids. The double-stranded nature of the DNA can be attributed to the twisting of the polymer backbone. It is also negatively charged and can easily bond with water molecules.
![]()
3. Nitrogenous Base
Through his observations, Phoebus Levene discovered that the genetic material is made up of four smaller sub-units distributed in equal quantities.
- These were later found to be the nitrogenous bases adenine, guanine, cytosine, thymine, and uracil. These five bases are further classified into two groups namely: pyrimidines and purines. Cytosine, thymine, and uracil are the pyrimidines while adenine and guanine are the purines.
- As its name suggest, A nitrogenous base is a molecular structure that contains nitrogen and acts as a base during a reaction.
![]()
Types of Nucleotide Bases
The bases are part of the DNA and RNA that serve as the storage of information and encodes for the phenotype, or the visible physical characteristic of an organism. The five known nucleotide bases are described below.
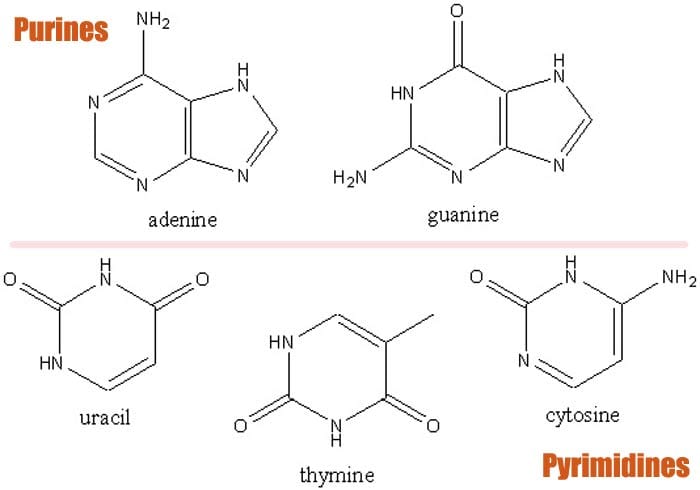
1. Adenine (Purines)
As alluded to earlier, adenine[7] belongs to the purines which are composed of a nitrogen group with six members of nitrogen ring attached to a ring with five nitrogen units. In DNA, adenine pairs with thymine whereas in RNA, it pairs with uracil.
Chemical formula for Adenine: C5H5N5.
- When fused with ribose, adenine forms the nucleoside adenosine. On the other hand, when fused with deoxyribose, it forms deoxyadenosine.
- This adenosine is an essential component of adenosine triphosphate (ATP) which is a unit of energy and is utilized during metabolism.
![]()
2. Guanine (Purines)
Also a purine molecule, guanine[8] is composed of a fused system of pyrimidine and imidazole ring with double bond conjugates. This bicyclic molecule is planar due to being unsaturated.
Chemical formula for Guanine: C5H5N5O.
- As a nucleoside, guanine is called as guanosine.
- Guanine pairs with cytosine.
![]()
3. Cytosine (Pyrimidines)
The next base is the cytosine[9] , a pyrimidine base that pairs with guanine. When combined with ribose, cytosine forms the nucleoside cytidine. This molecule can then be further phosphorylated to form phosphoric groups.
Chemical formula for Cytosine: C4H5N3O.
- Cytosine is very essential in cancer biology since its deamination alone is the main cause in the formation of cancer like leukemia.
![]()
4. Thymine (Pyrimidines)
Derived from the hydrolysis of deoxyribonucleic acid via catalytic reduction,thymine[10] is a pyrimidine base that pairs with adenine in DNA. The bond is secured with a hydrogen bond that stabilizes the structure of the nucleic acid.
Chemical formula for Cytosine: C5H6N2O2.
- In RNA, uracil replaces thymine at carbon five positions.
![]()
5. Uracil (Pyrimidines)
Uracil[11] is a pyrimidine that replaces thymine in RNA and therefore is the one that pairs with adenine. Through methylation, uracil can be converted into thymine.
Chemical formula for Cytosine: C4H4N2O2.
- In the body, uracil is used to synthesize various enzymes which are necessary for the correct functioning of cells.
![]()
Importance of Nucleotides

As building blocks of nucleic acids, nucleotides primarily aid in storing genetic information that will be later the basis for the manifestation of physical traits. Moreover, they also play roles in various biological and physiological processes. As molecules, they help in the transport of ATP and serve as biological catalysts in most reactions involving biomolecules.
![]()
It is quite impressive to know that no living organism in this world bears the same sequence of nucleotide bases. Because of such genetic diversity, organisms exhibit various phenotypes that help them survive.
What do you think could have happened if the nucleotide never evolved just like the way it is now?
![]()
Cite This Page
References
- [1] – “MLA CE Course Manual: Molecular Biology Information Resources (Genetics Review: Nucleotide)”. Accessed October 15, 2017. Link.
- [2] – Nucleotides. Accessed October 15, 2017. Link.
- [3] – “DNA Structure: The Discovery of Nucleic Acid Structure”. Accessed October 15, 2017. Link.
- [4] – “What are the Three Parts of a Nucleotide? | Albert.io”. Accessed October 15, 2017. Link.
- [5] – “Pearson – The Biology Place”. Accessed October 15, 2017. Link.
- [6] – “phosphate backbone | Learn Science at Scitable”. Accessed October 15, 2017. Link.
- [7] – “adenine | C5H5N5 – PubChem”. Accessed October 15, 2017. Link.
- [8] – “guanine | C5H5N5O – PubChem”. Accessed October 15, 2017. Link.
- [9] – “cytosine | C4H5N3O – PubChem”. Accessed October 15, 2017. Link.
- [10] – “thymine | C5H6N2O2 – PubChem”. Accessed October 15, 2017. Link.
- [11] – “uracil | C4H4N2O2 – PubChem”. Accessed October 15, 2017. Link.

















