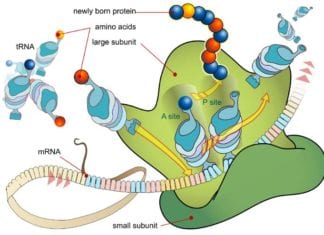
Biochemistry News of 2022: New studies reveal how salivary neutrophils can help in defense against COVID-19, how a drying droplet of peptide solution can aid in easier detection of Alzheimer’s, enzymes are used by biochemists to alter the communication process between brain cells, natural compounds with potential uses in agriculture and medicine is opened up by biosynthesis of cyanobacterin. This miracle protein can shield against chemical weapons.
Table of Contents
- Top 15 Biochemistry News of 2022
- 1. New study reveals that saliva has a novel COVID-19 defense mechanism (Japan, July 2022)
- 2. Scientists discover that neurodegenerative illnesses can be effectively identified using stain patterns from dried peptide solutions (Germany, July 2022)
- Top 10 Biochemistry News of 2020 – A Round-Up
- 3. Researchers have found a toxin that can destroy germs in a novel way (Canada, Sep 2022)
- 4. Researchers from various fields are using sound to comprehend biochemical processes better (USA, Feb 2022)
- 5. Biochemists use enzymes to alter the communication process between brain cells (USA, June 2022)
- Top 14 Biochemistry News, Innovations & Breakthroughs In 2017
- 6. Researchers find a cancer trigger that may lead to more effective medication therapy (USA, July 2022)
- 7. The discovery shows how to alter immune cells to combat cancer (UK, Oct 2022)
- 8. The structure of the enzyme reveals how the strigolactone hormone regulates plant development (USA, April 2022)
- 9. Successful cardiac muscle cell repair and regeneration by biochemistry researchers (USA, June 2022)
- 10. Researchers discover a novel mitochondrial enzyme that is similar to PARP (USA, Feb 2022)
- Top 25 Integumentary System Facts (Skin Fun Facts)
- 11. Researchers develop a method to program pH (USA, July 2022)
- 12. Mice’s enzyme-driven cognitive deterioration suggests a novel Alzheimer’s disease treatment target (USA, Nov 2022)
- 13. A new category of natural compounds with potential uses in agriculture and medicine is opened up by the biosynthesis of cyanobacterin (Germany, May 2022)
- 25 Mind-Blowing Biology Breakthroughs That Shaped Our World!
- 14. ‘Licencing’ dynamics of DNA replication revealed by groundbreaking study (Germany, Jan 2022)
- 15. Scientists have developed a protein that could shield against chemical weapons (USA, Sep 2022)
Top 15 Biochemistry News of 2022
Let’s take a glimpse at the research findings of 2022 in this field with broader perspectives.
1. New study reveals that saliva has a novel COVID-19 defense mechanism (Japan, July 2022)

The transmission and infection of the COVID-19 coronavirus occur through saliva and oral cells. Understanding how SARS-CoV-2 evades the body’s defense mechanisms prevents its spread. The onset and severity of COVID-19 infection vary with age, as does the quantity and quality of saliva, which significantly decrease in individuals. A group of researchers proposed that the innate immune system might play a role against SARS-CoV-2 infection.
- The transmission of SARS-CoV-2 occurs when the membrane of cells binds to the protein S1 on the viral envelope, specifically through the angiotensin-converting enzyme 2 (ACE2) receptor. Key routes of COVID-19 infection include saliva and oral cells.
- The study team has demonstrated that saliva from healthy, SARS-CoV-2-uninfected people affects S1 and ACE2 binding in a concentration-dependent way.
- They discovered four proteins that bind to ACE2 in saliva. Among these, neutrophil elastase and histone H2A significantly limit the interaction between S1 and ACE2. Positively charged proteins such as neutrophil elastase and H2A block ACE2’s negatively charged S1 binding site from interacting with the SARS-CoV-2.
- The study team also demonstrated that cationic polypeptides, including -poly-L-lysine, work just as well to stop S1 from attaching to ACE2. This study demonstrates that local bacteria trigger salivary neutrophils and continually release various proteins.
- Elastase and histone H2A are two proteins that help the body defend itself against SARS-CoV-2 infection by covering up the ACE2 receptor on host cells.
These discoveries will aid in developing strategies to stop not only COVID-19 infection but also future innate immune-level infections of people by unidentified viruses.
Suggested Reading:
Top 15 Biochemistry News of 2021
2. Scientists discover that neurodegenerative illnesses can be effectively identified using stain patterns from dried peptide solutions (Germany, July 2022)

When proteins or peptides fold incorrectly (misfold), their spatial structure is altered, leading to neurodegenerative disorders, including Alzheimer’s and Parkinson’s. This is the outcome of slight variations in the biomolecules’ chemical makeup. Researchers have devised an easy-to-use technique to identify such misfolding early in the disease.
- Proteins and peptides’ biological functions are governed by their biochemical structures. Small structural modifications can encourage the growth of illnesses.
- A single amino acid residue separates each of the amyloid beta (A-42) peptides, which are hereditary mutations of Alzheimer’s disease and play a significant part in the disease.
- Scientists have demonstrated that the stains left behind when drying drops of peptide solution on a solid surface can reveal important details about the main and secondary structures of peptides.
- An automated pipetting device accurately places the protein and peptide solutions on glass slides to achieve controlled and repeatable results. The slides’ surfaces were previously coated with a hydrophobic polymer.
- The researchers used polarisation microscopy to capture images to examine the intricate stain patterns from the dried droplets.
- After that, deep-learning neural networks were used to analyze the photos. The stain patterns left behind by amyloid beta peptides behave as precise fingerprints that reveal a peptide’s structural and spatial identification.
- The findings imply that a technique as straightforward as drying a droplet of peptide solution on a solid surface can be a marker for minute variations in the main and secondary structures of peptides.
With the help of this technique, Alzheimer’s variations can be identified quickly and with the highest resolution possible. Additionally, it is a reasonably easy procedure that allows for a quick and patient-friendly diagnosis because it does not call for complicated sample preparation. The technique is also promising for other uses in disease molecular identification and medical diagnostics.
Suggested Reading:
Top 10 Biochemistry News of 2020 – A Round-Up
3. Researchers have found a toxin that can destroy germs in a novel way (Canada, Sep 2022)
According to a recent study, the bacterial pathogen Pseudomonas aeruginosa, known to cause diseases acquired in hospitals like pneumonia, secretes a toxin that has evolved to eradicate other bacterial species.
- The research demonstrates that Pseudomonas aeruginosa, a bacterial pathogen known to cause hospital-acquired diseases, including pneumonia, secretes a toxin that has evolved to eradicate other bacterial species.
- Similar to humans, bacteria need healthy RNA to survive. This toxin enters its target, seizes a vital molecule to life, and then employs that molecule to obstruct regular functions. Given how many crucial pathways rely on functioning RNAs, it is a complete assault on the cell.
- To precisely grasp how this poison works at the molecular level, scientists have examined it for close to three years.
- The discovery was made after extensive testing on common toxin targets like protein and DNA molecules, followed by testing the toxin against RNA.
- The protein-targeting toxins released by other bacteria, such as those that cause cholera and diphtheria, have established precedents that our discovery defies.
- According to researchers, this breakthrough has much potential for further study that could ultimately result in brand-new discoveries that fight infection-causing germs.
Future antibiotic research can use the recently found weakness. A new generation of antibiotics may be possible due to the research.
Suggested Reading:
Top 15 Biochemistry Discoveries in 2018
4. Researchers from various fields are using sound to comprehend biochemical processes better (USA, Feb 2022)
Sonification, the use of sound to convey information, is used by a team of researchers from music, chemistry, and computer science to represent and better comprehend biochemical processes.
- The recent study led to a new understanding of how a protein might fold. The researchers experimented with sonification to study the physical mechanisms of protein folding.
- They worked together to create the digital audio software and hardware sound design system, Kyma, utilized by plenty of musicians and academics.
- They also produced an animated visualization with a sound that depicted a streamlined method of protein folding.
- They discovered that sonification enhanced and supported the visualizations and improved intuition for how proteins fold and misfold throughout time, even for experts.
- The research team ran simulations of proteins folding into a particular configuration using supercomputers.
- This process depends on a complicated pattern of many interactions. The simulation depicts the various routes the proteins can take to fold and when they misfold or become trapped in the incorrect shape, which is thought to be connected to several disorders, including Parkinson’s and Alzheimer’s.
- They connected features of proteins to audio parameters like pitch, timbre, loudness, and pan position using a variety of audio-mapping approaches.
- For instance, Taylor’s work uses various tones and instruments to depict the distinctive properties of each amino acid, such as whether it is hydrophobic or hydrophilic.
- The research team claimed that artists’ highly developed intuition contributed to developing the greatest tool for using sound to transmit information.
It offers a fresh perspective on how sound and music might aid in our understanding of the world. Musicians play a significant role. It could aid data analysis, protein folding instruction, and new scientific discoveries.
Suggested Reading:
Top 15 Biochemistry Discoveries of 2019
5. Biochemists use enzymes to alter the communication process between brain cells (USA, June 2022)
A recent study shows that enzymatic methods can alter the identity of synapses between neurons in vitro and in vivo. When sending information, the brain’s neurons, also known as cells, send electrical signals between each other through tiny, specialized junctions or synapses. Scientists are still unsure of the precise mechanisms by which the various synapses, such as “excitatory” or “inhibitory, ” develop between neurons.
- Biochemists have provided significant insight into this issue by demonstrating that the chemicals produced from synapses determine which synapses occur between neurons.
- By causing the neurons to express just a few genes that caused a cascade of changes in the synapses’ machinery, they could change synapses between excitatory and inhibitory kinds using only enzymes.
- Such a discovery could profoundly affect treating brain disorders brought on by errors in synaptic transmission and information exchange.
- According to their findings, neurotransmitters released from the presynaptic site (where the information is coming from) also appear to play a significant role in regulating which types of synapses form and where in contrast to what has previously been believed to be the sole source of the synaptic function being provided by cell-adhesion proteins expressed in the synaptic junction area.
- The CSU team demonstrated human neurons created from stem cells‘ capacity to create particular synaptic connections by carefully controlling the release of particular neurotransmitters.
The findings have implications for treating brain disorders brought on by problems in synaptic transmission and information processing.
Suggested Reading:
Top 14 Biochemistry News, Innovations & Breakthroughs In 2017
6. Researchers find a cancer trigger that may lead to more effective medication therapy (USA, July 2022)

Researchers have conclusively linked a human cancer trigger to the activity of a particular domain of proteins crucial to plant-microbe interactions, knowledge that had eluded scientists for decades.
- The plasminogen-apple-nematode, or PAN, domain, is connected to cell proliferation that fuels tumor growth in humans and defense signaling during plant-microbe interactions in bioenergy crops, researchers set out to demonstrate experimentally.
- The Oak Ridge National Laboratory (ORNL) team identified four cysteine residues essential to the hepatocyte growth factor (HGF) protein’s ability to function and observed their behavior in human cancer cell lines. They discovered that altering any of those amino acids prevented the HGF-c-MET signaling pathway, which is abnormally amplified in cancer cells, from causing them to increase and spread.
- Since cysteine residues are known to serve various purposes, researchers also tested a variety of other cysteines present in the protein at random. They discovered none had the same effect on inhibiting HGF-c-MET signaling.
- The scientists reported in the study that altering the four essential cysteines did not impact the protein’s overall structure and just blocked the cancer signaling pathway.
- One of the most difficult aspects of creating new cancer medicines is disrupting the appropriate signal. Engineering chemicals to interfere with a complete protein is highly challenging.
- Knowing which individual amino acids in that protein to target is a significant development. You only need to seek these four specific residues; you don’t need to search for the complete protein.
- The team’s ability to forecast outcomes by combining its knowledge of plant biology and biochemistry with genetics, computational biology, supercomputing, and access to the CRISPR/CAS-9 gene editing tool is demonstrated by discovering those critical residues.
The discoveries open up a new path for creating targeted pharmacological therapy to treat a range of malignancies, including those that start in the stomach and breast. The discovery may lead to treatments for other illnesses, such as blocking the malaria parasite’s infection pathway in mosquitos to reduce their ability to transmit the disease. It may also help combat the HLB virus, which kills citrus trees in Florida and California, by focusing on the Asian citrus psyllid insect that disseminates it.
Suggested Reading:
Top 25 Immune System Fun Facts
7. The discovery shows how to alter immune cells to combat cancer (UK, Oct 2022)
Melanoma, a type of skin cancer that’s both dangerous and challenging to treat, has shown results in responding to a unique approach aimed at reprogramming our immune cells. This innovative method aims to reduce or eliminate the size of cells. Researchers have put forward a strategy that targets not early-stage precancerous cells but also advanced tumor cells.
- The researchers have shown that they successfully transformed cells (also known as white blood cells) into a more effective state for suppressing the growth of melanoma cells. They achieved this by utilizing capsules called protocells.
- These protocells are designed to deliver reprogramming substances, which are then absorbed by the cells.
- They demonstrated that human and animal immune cells could do this. The research presents a promising new target for treating cancer immunotherapies. It is the first to examine a protocell’s ability to carry payloads for transforming immune cells.
- Immune cells can monitor any tissue site in the body for the emergence of pre-cancerous cells. However, when immune cells come into contact with cancer cells, the cancer cells frequently manipulate them and instead tend to feed them, promoting the spread of the disease.
- The experiment was repeated using an in vitro assay with primary human immune cells to examine the viability of employing protocells to deliver ‘reprogramming’ anti-miR223 payloads in humans.
- The experiment showed that the protocells could successfully transfer and reprogram human immune cells to become more persistently pro-inflammatory and possibly anti-cancer.
Results highlight the therapeutic advantages of leveraging host immunity to destroy cancer and show that it is possible to reprogram innate immune cells using protocells.
Suggested Reading:
Explore Different Types of Nerve Cells
8. The structure of the enzyme reveals how the strigolactone hormone regulates plant development (USA, April 2022)

Plants rely on systems to detect and react to their surroundings, constantly adjusting their growth and structure to meet the demands of a dynamic environment. Recent studies shed light on how proteins play a role in targeting and breaking down plant hormones involved in signaling pathways.
- The paper results from a study that revealed structural and molecular alterations in the MAX2 (or D3) ubiquitin ligase enzyme.
- It was discovered that MAX2 could engage the strigolactone sensor D14 and target the DNA transcriptional repressor complex D53 for eradication in locked and unlocked versions. All eukaryotes include ubiquitins, tiny proteins that “tag” other proteins for cellular apoptosis.
- Researchers employed a strategy combining cutting-edge structural biology, biochemistry, and plant genetics to unlock MAX2 and better understand its molecular dynamics in plants.
- Arabidopsis MAX2 enzyme was systemically mutated using structure-guided methods, resulting in a MAX2 stranded in an unlocked state.
- They discovered that MAX2 may target the repressor proteins in the unlocked confirmation and biochemically mark them with tiny ubiquitin proteins, designating them for destruction.
- A vast gene network that controls shoot branching, root architecture, leaf senescence, and symbiosis with fungi is activated by removing these repressors. The enzyme must relock to deliver these repressors to the proteasome disposal complexes.
- The group also demonstrated that MAX2 targets the strigolactone sensor itself after it is locked, restoring the system to its initial condition in addition to the repressor proteins.
- Finally, a metabolite of an organic acid that may immediately cause the conformational flip was discovered to be the key to the lock.
This is the first study to establish a primary metabolite as a direct novel regulator of this type of ubiquitin ligase enzyme, which will open new lines of research beyond the implications for plant signaling.
Suggested Reading:
Top 15 Botany News of 2021
9. Successful cardiac muscle cell repair and regeneration by biochemistry researchers (USA, June 2022)
Researchers have developed a groundbreaking technique that, after a heart attack or myocardial infarction as it is medically known, regenerates heart muscle cells in mice and repairs them.
- The innovative method created by the study team delivers mutant transcription factors, which are proteins that regulate the translation of DNA into RNA, to mice hearts using synthetic messenger ribonucleic acid (mRNA).
- To boost the replication of cardiomyocytes, or heart muscle cells, separated from mice hearts, the researchers showed that two mutant transcription factors, Stemin and YAP5SA, cooperate. On tissue culture dishes, these in vitro experiments were carried out.
- Stemin activates Cardiomyocytes’ stem cell-like characteristics, and YAP5SA operates by encouraging organ growth, which leads the myocytes to multiply even more.
- The group will also present data showing that Stemin and YAP5SA restored injured mouse hearts in vivo. Notably, myocyte nuclei multiplied at least 15 times within 24 hours after receiving those transcription factors via cardiac injections.
- The outcomes were astounding when both transcription factors were administered into infarcted adult mouse hearts.
- Within a day, the lab discovered that cardiac myocytes multiplied rapidly. Over the following month, hearts were repaired to almost normal cardiac pumping function with minimal scarring.
- Synthetic mRNA has the added advantage of disappearing in a matter of days compared to viral distribution, which raises many biosafety issues because it is difficult to halt them, claims Xiao. On the other hand, mRNA-based delivery soon degrades and vanishes.
Less than 1% of adult cardiac muscle cells can regenerate, making the discoveries all the more significant. This discovery could lead to the development of a potent treatment strategy for managing human heart disease.
Suggested Reading:
History of Immunology
10. Researchers discover a novel mitochondrial enzyme that is similar to PARP (USA, Feb 2022)
The mitochondria produce most of the chemical energy required to fuel cell biochemical reactions. This organelle has been linked to the identification and functioning of an ADP-ribosyltransferase enzyme. Enzymes called ADP-ribosyltransferases are involved in the modification of other proteins.
- This brand-new mitochondrial enzyme, NEURL4, shares properties with PARP1, a nuclear enzyme known for its crucial function in DNA damage repair and the control of gene expression.
- The scientists first conducted in-vitro enzymatic experiments to define NEURL4’s activity. Then, using a gene editing system, they created a cell line in which NEURL4 was deleted.
- After that, scientists used mass spectrometry to compare the alterations in mitochondrial proteins in wild-type (“normal“) cells and cells lacking NEURL4.
- According to the researchers, this method allowed them to pinpoint several mitochondrial proteins as NEURL4 targets, including mitochondrial LIG3, a protein involved in restoring damaged mitochondrial DNA.
- This method also allowed the researchers to confirm that NEURL4 activity is necessary to preserve mitochondrial DNA (mtDNA) integrity.
According to the researchers, further investigation into the effects of NEURL4-mediated changes of particular mitochondrial proteins on mitochondrial functioning may reveal novel regulatory mechanisms that might be targeted as potential therapeutic targets. The therapy of male infertility could benefit from a deeper comprehension of NEURL4 biology.
Suggested Reading:
Top 25 Integumentary System Facts (Skin Fun Facts)
11. Researchers develop a method to program pH (USA, July 2022)

Its pH or proton concentration determines the acidity of a solution. However, localizing pH is difficult because protons in a water-based solution spread quickly. By producing a dense array of microsites where the concentration of protons is 100 to 1000 times higher than the average in the rest of the solution, researchers have devised a method to adjust pH at the local level.
- It was made possible by a collection of specialized micrometer-scale electrochemical cells built on and controlled by a semiconductor integrated circuit chip.
- The semiconductor chip is in direct contact with a water-based solution of quinone molecules. It has 256 electrochemical cells on its surface. Each cell has two concentric metallic rings that resemble a bullseye.
- The inside ring introduces a current into the solution to electrochemically create protons from quinone molecules. While trying to disperse, these locally generated protons are neutralized close to the exterior ring, which electrochemically converts quinone molecules into base molecules by drawing current from the solution.
- This results in the locally generated protons being confined in and around the bullseye’s center, which lowers the pH and creates an acidic microenvironment.
- This spatial-selective pH programming is made possible by the distinctive cell structure the researchers established on the semiconductor electronic chip.
- By altering the currents of the concentric rings of each electrochemical cell, the device can localize and accurately tune pH and use on-chip pH sensors dispersed across the electrochemical cell array to monitor pH continuously.
This study makes various pH-regulated chemistry, including biomolecular synthesis, applicable in a high-throughput setting. The approach opens the door for many other biological applications, such as developing synthetic biology-based enzymes and oligo libraries for diagnostics.
Suggested Reading:
Introduction To Fermentation Biology
12. Mice’s enzyme-driven cognitive deterioration suggests a novel Alzheimer’s disease treatment target (USA, Nov 2022)
A mutation that enhances the activity of the protein kinase C (PKC)-alpha enzyme has been linked to biochemical, cellular, and cognitive deficits in mice, making it a prospective therapeutic target in Alzheimer’s disease research.
- Researchers discovered that the slight increase in PKC could cause biochemical, cellular, and cognitive deficits in mice comparable to those seen in Alzheimer’s disease (AD), which affects humans.
- Multiple research teams worked together to create a mouse model with the PKC M489V mutation and then analyze its biochemistry and behavior over an additional 1.5 years (equivalent to roughly 55 years in human aging) to determine its role in AD.
- After three months, the mutant mice’s brains’ protein phosphorylation levels were significantly different from those of the wild-type control mice’s brains, demonstrating that the regulation of neuronal proteins was off.
- The hippocampus neurons of the mice had various cellular alterations by the time they were 4.5 months old, including synaptic depression and a decreased density of dendritic spines.
- The mice displayed reduced performance in behavioral tests of spatial learning and memory at 12 months, which is unmistakable proof of cognitive deterioration.
- It was unexpected to learn that even a little increase in PKC activity was sufficient to induce the mice version of the Alzheimer’s phenotype. The researchers also examined the frontal cortex of human brains from deceased AD patients and control subjects for protein levels.
- PKC levels in the brains of AD patients were up 20%. Another indication that PKC activity was increased in the human AD brain was the nearly four-fold increase in phosphorylation of a known PKC substrate in these brains.
Several PKC pharmacological inhibitors have already been created for cancer treatment but could also be used to treat AD. Future pharmacological research may concentrate on strategies to inhibit PKC at synapses specifically.
Suggested Reading:
Are Enzymes Proteins?
13. A new category of natural compounds with potential uses in agriculture and medicine is opened up by the biosynthesis of cyanobacterin (Germany, May 2022)
The cyanobacteria Scytonema Hofmann produces the natural product cyanobacterin in modest amounts in Nature. Researchers have succeeded in understanding the biosynthetic processes for this process. Additionally, they made a new class of carbon-carbon bond-making enzymes during the process.
- Combining cutting-edge tools from bioinformatics, synthetic biology, enzymology, and (bio)chemical analytics allowed for the completion of this work.
- The main discussion topic was the production process for the cyanobacterin carbon skeleton’s core. The suspected genes for this were first cloned using the “Direct Pathway cloning” (DiPaC) technique, and they were subsequently turned on in the model organism e.coli as a cell factory.
- DiPaC, a brand-new synthetic biology technique previously created in the lab, enables the speedy and effective transfer of whole natural products’ biosynthetic pathways into recombinant host systems.
- The research team also produced all important enzymes in the host organism E.coli, isolated them, and then examined the function of each enzyme to analyze the crucial individual steps of cyanobacterin production.
- They discovered a group of enzymes known as furanolide synthases throughout the process. These have an unusual mechanism for catalyzing the formation of carbon-carbon bonds.
- These enzymes were effective in vitro biocatalysts in subsequent research on furanolide synthases, making them desirable for biotechnological applications.
We now have an enzymatic tool in the form of furanolide synthases that will enable us to create future bioactive chemical synthesis processes that are more ecologically friendly. The research sets the path for the thorough development of an intriguing class of natural products with potential uses in agriculture and medicine.
Suggested Reading:
25 Mind-Blowing Biology Breakthroughs That Shaped Our World!
14. ‘Licencing’ dynamics of DNA replication revealed by groundbreaking study (Germany, Jan 2022)

A crucial mechanism that takes place during cell division and is probably the cause of DNA damage in some situations, including cancer, has been made clear by a recent scientific study.
- The researchers created a sophisticated experimental setup to investigate the “origin licensing” process. Cells use this procedure to control or “license” the replication of their genomes during cell division.
- They demonstrated in particular how these dynamics differ in the two fundamental states of genomic DNA, the “euchromatin” state, which is relatively loose and open for gene activity, and the “heterochromatin” state, which is wound more tightly to silence gene activity, and how these differences bring about different risks of DNA damage during replication.
- Origin licensing takes place in the G1 phase, the first and most preliminary stage of cell replication. It entails several sites on the DNA in chromosomes where DNA-copying is to begin being attached by sets of specialized enzymes.
- The enzymes grant permission for DNA cloning to prevent cells from duplicating their genomes more than once.
- This discovery at least suggested that the highly compacted DNA in the cell genome would not always be fully licensed for replication, potentially leading to significant mutations during replication and even cell death in dividing cells with an abnormally short G1 phase.
- There was considerably more under-replication and DNA damage in heterochromatin parts of the cells’ genomes than in euchromatin regions as a result of artificially shortening the G1 phase in test cells, confirmed this theory.
The discoveries could, for instance, assist in explaining why specific regions of the genome in particular cancer cells are more likely to sustain DNA damage during replication. The work also establishes the experimental platform of the researchers as a tool for additional research on origin licensing dynamics and genomic instability, research that could one day result in new cancer-fighting treatments, among other things.
Suggested Reading:
From mRNA To Protein: Overview of Translation Biology
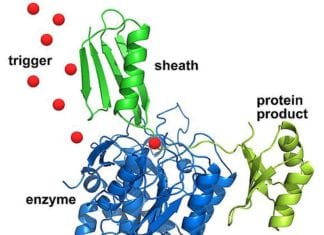
15. Scientists have developed a protein that could shield against chemical weapons (USA, Sep 2022)

A team of scientists has created a synthetic protein that swiftly recognizes molecules of a deadly nerve toxin, an agent designated as a weapon of mass destruction by the United Nations.
- The team produced the protein through a unique design on fast computers in the labs. In this case, an artificial protein that attaches to a chemical target, the nerve toxin VX, has been created.
- VX is the most lethal and quickly acting of all the known chemical warfare agents. It has no taste or odor and was created by humans. It functions by assaulting the neurological system, resulting in muscle paralysis and rapid asphyxial death.
- The researchers created the protein with a hollow in the middle that precisely matched VX’s physical characteristics and chemical makeup.
- A protein made from the design was created by collaborators, purified, and sent overnight on ice to a laboratory authorized to test chemical weapons. Within 24 hours, the protein was tested against VX there.
- VX was buried inside the cavity we created when the protein underwent a significant shape change. The signal that could be linked to a sensing device is this shape change. The protein is 1, 000 times more sensitive than existing technology to detect VX.
- Additionally, the protein doesn’t cause false positives, which happen when modern sensors mistakenly detect chemicals that aren’t nerve agents but are similar, like some pesticides.
Developing a new generation of biosensors, treatments, and diagnostics should be possible thanks to the design methodology described here. This research opens the door for VX-specific treatments that could be used in chemical warfare.
Suggested Reading:
Top 38 Fun Facts About The Nervous System
This series of biochemistry news gives us a detailed account of the recent development in this field. Starting with the role of Elastase and histone H2A – two proteins that help the body defend itself against SARS-CoV-2 infection by covering up the ACE2 receptor on host cells, the discovery that neurodegenerative illnesses can be effectively identified using stain patterns from dried peptide solutions, Mechanisms of strigolactone hormone regulating plant development, the effects that NEURL4-mediated changes of particular mitochondrial proteins have on mitochondrial functioning may reveal novel regulatory mechanisms that might be targeted as potential therapeutic targets, etc.
With these developments as the foundation, we expect more exciting findings in 2023.
![]()